Projet EPICOREM
Context, position and objectives
-
Objectives, originality and novelty of the project
-
Coronaviruses have a wide spectrum of hosts including a great number of both mammalian and avian species. The pathologies for which they are responsible are essentially respiratory and enteric. Their severity is variable with respect to the host and its immune status. The first animal coronaviruses were described in the 1930s: the IBV or “infectious bronchitis virus” in domestic fowl, the “transmissible gastroenteritis coronavirus” in domestic pigs, and the MHV or “mouse hepatitis virus”. These viruses, responsible for serious pathologies in these species, were first studied in veterinary science. Human coronaviruses OC43 and 229E were described in the 1960s, but have attracted limited interest because of the mild respiratory symptomatology which they were linked to.
-
The evolutive and emerging potential of coronaviruses came into the limelight during the 2002-2003 SARS (Severe Acute Respiratory Syndrome) pandemic. The event of the SARS pandemic claims several features that are unique to it: it was the first of the 21st century pandemics; it was the first pandemic to benefit from Internet networking along with tight international collaboration between research laboratories; and the first to be brought under control thanks to the rapid launching of sound health policy.
-
-
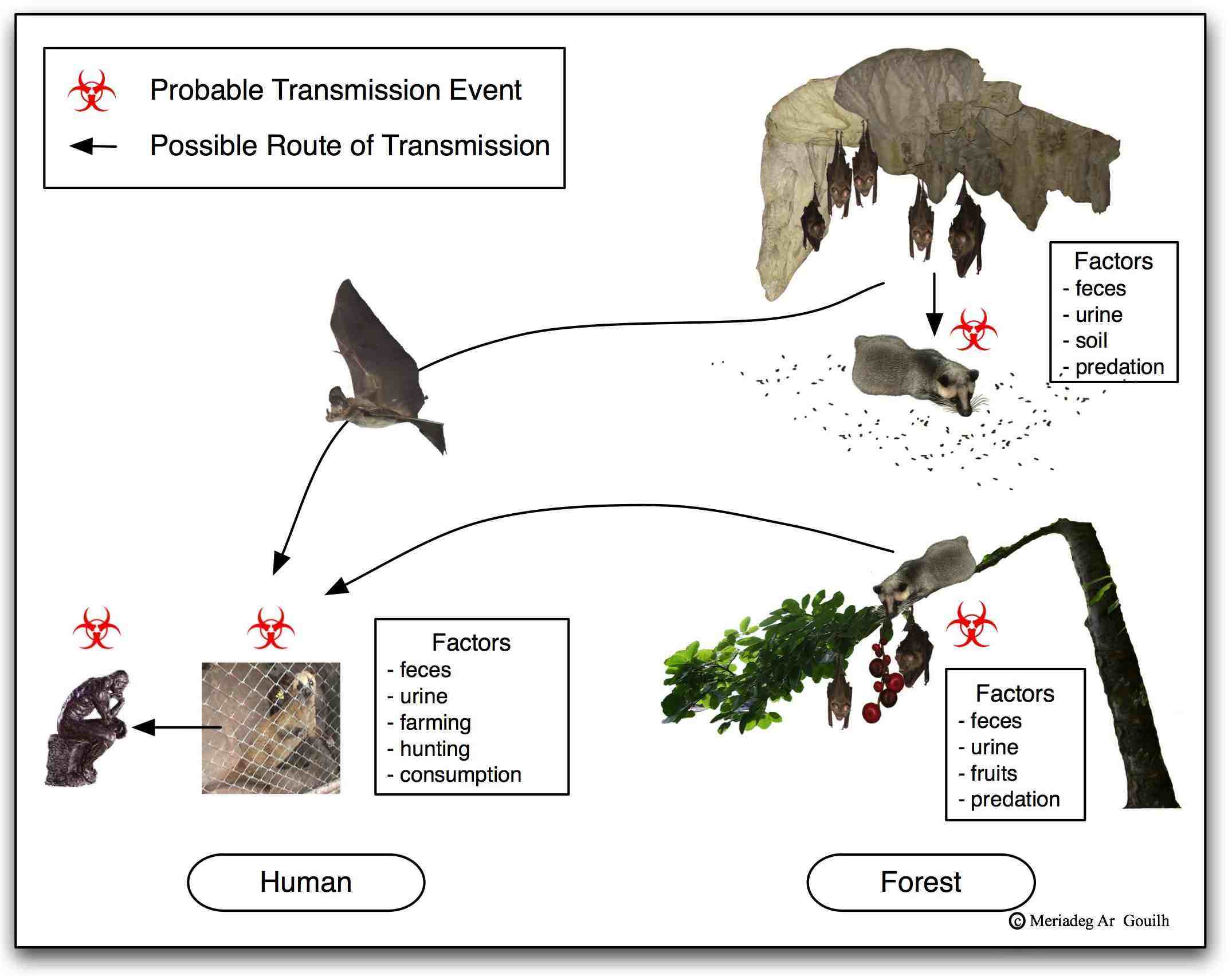
The long-term effects of Human Immunodeficiency Virus (HIV) pandemic infection constantly remind us of the permanent risk of emergence of zoonotic pathogens spreading from their animal reservoirs to humans, and the need to be brought under control as quickly as possible. The economic cost of such control is not limited to expenditures in human and animal health; it is widely encumbered by the suspension of social life, including trade. Thus, the economic cost of the SARS pandemic was estimated at between 30 and 50 billions USD, though the virus circulated only 8 months in the human population and infected less than 9,000 individuals on the planet. A deeper understanding of the ecology of zoonotic infections, especially at the interface between humans and other animal species, proves necessary for improving prevention measures to the risk of emergence. Such thinking is an integral part of the new “One Health” approach that incorporates broader, more holistic perspective to the continuum between ecosystems. This approach allows us to bring forth levers of preventive action, namely rapid detection of an “abnormal” phenomenon and the implementation of a rapid and adequate response (figure 1).
-
Following the SARS pandemic, two other coronaviruses that infect humans (NL63 and HKU1) were identified. Along with Paramyxoviridae and influenza viruses, the Coronaviridae family belongs to the three viral families most closely monitored in the context of emerging diseases. The identification in September 2012 of the MERS-CoV (Middle-East Respiratory Syndrome Coronavirus), responsible for a SARS-like syndrome and acute renal failure in humans, provides a clear warning that this risk is of current concern. Nine cases, to date, have been reported and confirmed from a virological viewpoint, all from the Arabic peninsula. This new coronavirus is genetically close to some bat coronaviruses, and it evokes the problematic question of whether it is a matter of a sporadic crossing of the species barrier without major consequences in epidemic terms, or if the virus will acquire the ability to effectively be transmitted from human to human, and to constitute a real danger of successful emergence.
- The objective of the present project arises from the context of this investigation. We propose a horizontal study of coronaviruses – viruses with a high evolutive potential -, and their impact on different animal populations as potential sources of human infection. The primary aim of this project is a transversal investigation of the circulation, the diversity, and the transmission dynamics of coronaviruses in French territory, between the different ecosystems represented, from wildlife (bats, rodents, wild boar, lagomorphs), domestic carnivores (dogs, cats), livestock (cattle, horses, pigs, rabbits), and the avian reservoir to Human.
- The first goal is to measure the presence and the impact of coronaviruses in the different ecosystems shared by these viruses (seroprevalence, molecular detection, amplification in cell culture).
- The second is to perform a molecular and phylogenetic analysis of isolated cultures taken from different ecosystems.
- The third objective is to study the key factors of transmission and the adaptation of coronaviruses under different evolutive constraints.
- The originality of this project is found in its horizontal approach to the ecology of coronaviruses, from wildlife (as yet unexplored in our territory) to Human. This approach imposes a multidisciplinary character on the researchers involved. In this way, our project brings together epidemiologists, zoologists, molecular biologists, evolutionary biologists and virologists working in the fields of veterinary and human medicine. Most of them hold expertise in the biology of coronaviruses.


Share this Post

